
HIV NPEP Access Toolkit > HIV Risk Assessment
HIV Risk Assessment
Introduction
HIV seroconversion has occurred among persons whose only known risk factor was sexual assault or sexual abuse; however, the frequency of this occurrence is likely low. In consensual sex, the per-act risk for HIV transmission from vaginal intercourse is 0.08%, and for receptive anal intercourse, 1.38%. The per-act risk for HIV transmission from oral sex is substantially lower. Specific circumstances of an assault (e.g., bleeding, which often accompanies trauma) might increase risk for HIV transmission in cases involving vaginal, anal, or oral penetration. Site of exposure to ejaculate, viral load in ejaculate, and the presence of an STI or genital lesions in the assailant or survivor also might increase risk for HIV acquisition (Centers for Disease Control and Prevention [CDC], 2021b).
PEP with a 28-day course of zidovudine was associated with an 81% reduction in risk for acquiring HIV in a study of healthcare workers who had percutaneous exposures to HIV-infected blood. On the basis of these results and results from animal studies, PEP has been recommended for healthcare workers who have occupational exposures to HIV. These findings have been extrapolated to nonoccupational injecting drug and sexual HIV exposures, including sexual assault. The possibility of HIV exposure from the assault should be assessed at the initial examination; survivors determined to be at risk for acquiring HIV should be informed about the possible benefit of PEP in preventing HIV infection. Initiation of PEP as soon as possible after the exposure increases the likelihood of prophylactic benefit (CDC, 2021b).
Multiple factors affect the medical recommendation for PEP and affect the assault survivor’s acceptance of that recommendation. These factors include the likelihood of the assailant having HIV, any exposure characteristics that might increase the risk for HIV transmission, the time elapsed after the event, and the potential benefits and risks associated with PEP. Determination of the assailant’s HIV status at the time of the post assault examination is usually not possible. Therefore, healthcare providers should assess any available information concerning the characteristics and HIV risk behaviors of the assailant (e.g., being an MSM or using injecting drugs), local epidemiology of HIV/AIDS, and exposure characteristics of the assault. When an assailant’s HIV status is unknown, determinations about risk for HIV transmission to the survivor should be based on whether vaginal or anal penetration occurred; whether ejaculation occurred on mucous membranes; whether multiple assailants were involved; whether mucosal lesions were present in the assailant or survivor; and any other characteristics of the assault, survivor, or assailant that might increase risk for HIV transmission (CDC, 2021b).
The information above, as well as the risk assessments below, can serve to guide conversations between the patient and healthcare provider as well as inform clinical decision-making. In summary, the medical-forensic history, clinical examination findings and any known information regarding the assailant’s HIV status will be essential in creating the final treatment plan.
Tools for Evaluating HIV Risk After Sexual Assault
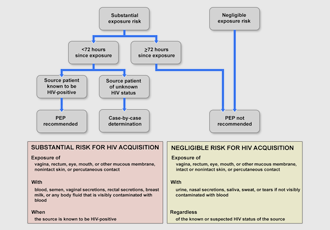
Algorithm for evaluation and treatment of possible non-occupational HIV exposures (AIDS Education and Training Center [AETC], 2021; Dominguez et al., 2016, p. 23).
Source: https://www.cdc.gov/std/treatment-guidelines/sexual-assault-adults.htm

nPEP Quick Guide for Providers
Assessment, treatment, and follow-up recommendations for people with known or potential exposures to HIV and other infections from the AIDS Education and Training Center (AETC).

HIV Seroconversion Risk Stratification Tool
Assessment and treatment recommendations for individuals with known or potential exposure to HIV after a sexual assault. (Developed by the University of Pittsburgh Medical Center.)

This AETC Program app supports health care providers with point-of-care tools for HIV screening, prevention, and care. Free to download on Apple and Android phones.
The Pregnant Patient
All forensic programs and facilities should have guidelines or policies created to address this special population.
Because pregnancy has been demonstrated to increase susceptibility to sexual HIV acquisition, PEP can be especially important for people who are pregnant. If the person exposed to HIV is pregnant, expert consultation should be sought. In general, however, PEP is indicated at any time during pregnancy when a significant exposure has occurred, despite a possible risk to the pregnant patient and the fetus. The recommended PEP regimen remains the same (CDC, 2021a, p. 9).
No trials have been conducted to evaluate use or the maternal or fetal health effects of short-term (i.e., 28-day) antiretroviral use as nPEP among pregnant women without HIV infection. However, clinical trials have been conducted and extensive observational data exist regarding use of specific antiretrovirals during pregnancy among HIV-infected women both when initiated as treatment for health benefits to the women and when initiated to reduce mother-to-child HIV transmission. Although duration of antiretroviral use during pregnancy has varied in these trials, it often spans months of pregnancy. Only ZDV is specifically approved for use in pregnancy, but as a result of data from clinical trials, other antiretroviral drugs have been reported to have short-term safety for pregnant women and their fetuses, and therefore can be considered for nPEP in women who are or who might become pregnant (Dominguez et al, 2016, pp. 18-19).
Additional assistance can be found by calling the HIV Clinician hotline at 888-448-4911, Monday through Friday from 9 a.m. to 8 p.m. (EST) and 11 a.m. to 8 p.m. (EST) weekends & holidays.
Note: Further information regarding treatment options for the pregnant patient can be found by clicking on the Prophylaxis and Follow-Up tab.
